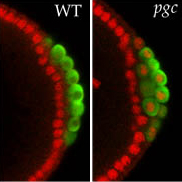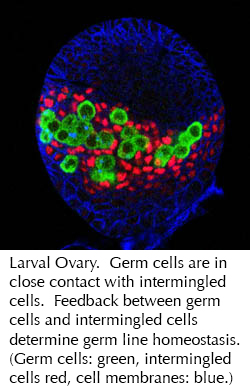
In order to gain a more complete understanding of the regulatory networks governing stem cell self-renewal and differentiation, we recenlty performed an unbiased, transcriptome-wide in vivo RNAi screen in Drosophila germ line stem cells (GSC). Characterization of cellular defects during early stages of ovarian GSC self-renewal and differentiation allowed the lab to sort ~650 “hits” into phenotypically and functionally meaningful groups. Bioinformatic analysis unveiled a comprehensive set of networks regulating GSC maintenance, survival or differentiation. This analysis revealed an unexpected role for ribosomal assembly factors in controlling stem cell cytokinesis. We found that the transition from self-renewal to differentiation relies on enhanced ribosome biogenesis accompanied by increased protein synthesis. In addition, we discovered a specific requirement for the mitochondrial ATP synthase in GSC differentiation. Surprisingly, while the screen identified almost every subunit of the ATP synthase, knockdown of other members of the oxidative phosphorylation system did not disrupt differentiation. We found that ATP synthase promotes the maturation of mitochondrial cristae during differentiation through dimerization and specific up-regulation of the ATP synthase complex. Immature mitochondria have been observed in many stem cell systems, and have been attributed to the use of glycolysis rather than oxidative phosphorylation as an energy source in stem cells. Then, when stem cells differentiate and mitochondria mature, this was interpreted as reflecting a need to switch to oxidative phosphorylation. The new data suggest that mitochondrial maturation may have oxidative phosphorylation independent roles for differentiation such as maintenance of mitochondrial DNA, calcium homeostasis and cell death. Collectively, this systematic analysis details the extensive genetic networks that control stem cell homeostasis and allows us now to connect these networks functionally in the context of a niche-stem cell system.
Research Areas:
|
|
|
|
|
|